ATOMIC STRUCTURE
An atom is the smallest part of an element that can exist and it consists of subatomic particles called protons, neutrons and electrons.
Subatomic particle Relative mass Relative charge Position
Proton 1 +1 Nucleus
Neutron 1 0 Nucleus
Electron 1/1840 -1 Shell
Deflection in electric field:
Electronic Configuration of elements:
Subatomic particle Relative mass Relative charge Position
Proton 1 +1 Nucleus
Neutron 1 0 Nucleus
Electron 1/1840 -1 Shell
Deflection in electric field:
When a beam of subatomic particles is passed through an electric field,1. Neutrons will pass through undeflected since they have no charge2. Protons, being positively charged, will show a slight deflection towards the negative plate3. Electrons, being negatively charged will show a larger deflection towards the positive plate.The deflection of electrons > the deflection of protons since electrons have a lower mass (are lighter) than protons.
Atomic number is the number of protons in an atom. It is equal to the number of electrons.
Mass number is the sum of protons and neutrons in an atom. It is also called the nucleon number.
Isotopes are atoms of the same element with the same atomic number but different mass number, i.e they have the same number of protons and electrons, but a different number of nucleons. In other words, they have the same physical properties but different chemical properties.
Electrons in an atom are found in shells of energy levels, numbered 1,2,3,4 starting from the nucleus. These numbers are called the principle quantum numbers.
Each shell has a number of subshells whereby the number of subshells= principle quantum number
The subshells are named s,p,d,f
Subshells are a number of orbitals with different energy values.
An orbital is a region in which there is greatest probability of filling particular electrons.
Each orbital has its own specific 3D shape.
Square boxes can be used to represent an orbital:
Electrons are accommodated in the lowest energy subshell first, that is s.
2 electrons in the same orbitals must have opposite spins so that their magnetic attraction resulting from the opposite spin counterbalances the electric repulsion resulting from their identical negative charges. (Like charges repel, unlike charges attract)
Tip: To remember the order of increasing energy values of orbitals, the following method can be used (just draw parallel lines as shown)
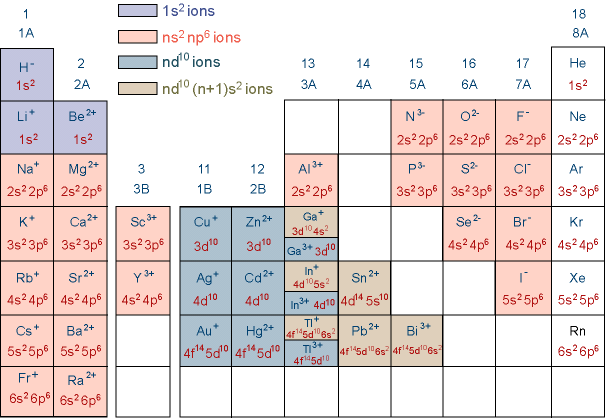
Electronic Configuration of elements:
Electronic Configuration of ions:
To form cations, electrons are removed from the highly energetic subshells first.
To form anions, electrons are added to the highly energetic subshells first.
Ionisation Energies:
The first ionisation energy is the energy required to remove one mole of electrons from one mole of gaseous atom to form one mole of unipositive gaseous ions.
eg Na(g) ------> Na+(g) + e 1st Ionisation of sodium
The second ionisation energy is the energy required to remove one mole of electrons from one mole of gaseous unipositive ions to form one mole of gaseous bipositive ions.
eg Na+(g) --------> Na2+ (g) + e 2nd Ionisation of sodium
Factors affecting ionisation energy:
1. Nuclear Charge: The bigger the nuclear charge, the greater is the force of attraction on the electron to be removed and hence the larger is the amount of energy required to remove it.
2. Size of atom:The larger the atom, the further is the electron to be removed from the nucleus. Hence the force of attraction exerted on the electron by the nucleus is smaller. Therefore a smaller amount of energy is needed to remove that electron. Furthermore as size of atom increases, number of shells increases hence increasing the shielding effect exerted by the inner electrons upon the eletron to be removed.
Therefore:
1.Increase in nuclear charge increases 1st ionisation energy
2.Increase in size of atom decreases 1st ionisation energy
3.Increase in shielding effect decreases 1st ionisation energy
Conclusion:
1.Down a group, number of shells increases therefore size of atom as well as shielding effect increases, hence Ionisation energy decreases down a group.
2.Across a period, nuclear charge increases. Therefore ionisation energy increases across a period.
Exceptions:
1. The 1st Ionisation energy of aluminium is unexpectedly lower than that of magnesium.
Reason: In aluminum, the electron to be removed is from a more energetic subshell (3p) which is further away from the nucleus as compared to the electron to be removed from magnesium (3s).
2.The 1st Ionisation energy of sulfur is unexpectedly lower than that of phosphorus.
Reason: In sulfur, the interelectronic repulsion between the paired electrons in the 3p orbital facilitated the removal of the electron. Such repulsion is absent in phosphorus.



No comments:
Post a Comment